"How many patients actually fit the inclusion criteria for a given study?"
It’s a question that determines protocol design, feasibility, budget, timelines, and ultimately the success or failure of a clinical trial. Yet, in most cases, trial sponsors and sites have no easy way to answer it.
In-Site Insights™ is an analysis which runs within Bitfount's secure on-premise AI platform and is designed to extract actionable insights from a clinic’s existing and routinely collected imaging and EHR data. The analysis occurs entirely within the clinic’s environment, with no patient data leaving the premises or being transferred to external servers for analysis.
Through In-Site Insights™, a clinic can determine, with quantitative certainty, how many patients exhibit GA lesions of a specific size, growth rate, or foveal involvement, parameters that map directly to trial eligibility. This precision enables clinics to engage with sponsors and CROs not as passive recipients of studies, but as data-informed collaborators capable of maximising their recruitment potential.
The platform leverages Altris AI’s OCT analysis algorithms to identify and quantify biomarkers across a range of retinal conditions, including GA, CNV, and dry/wet AMD, etc. The analysis is compatible with OCT scans captured via any device, and the output is a comprehensive, automatically generated report detailing the clinic’s patient population profile, disease prevalence, and imaging characteristics.
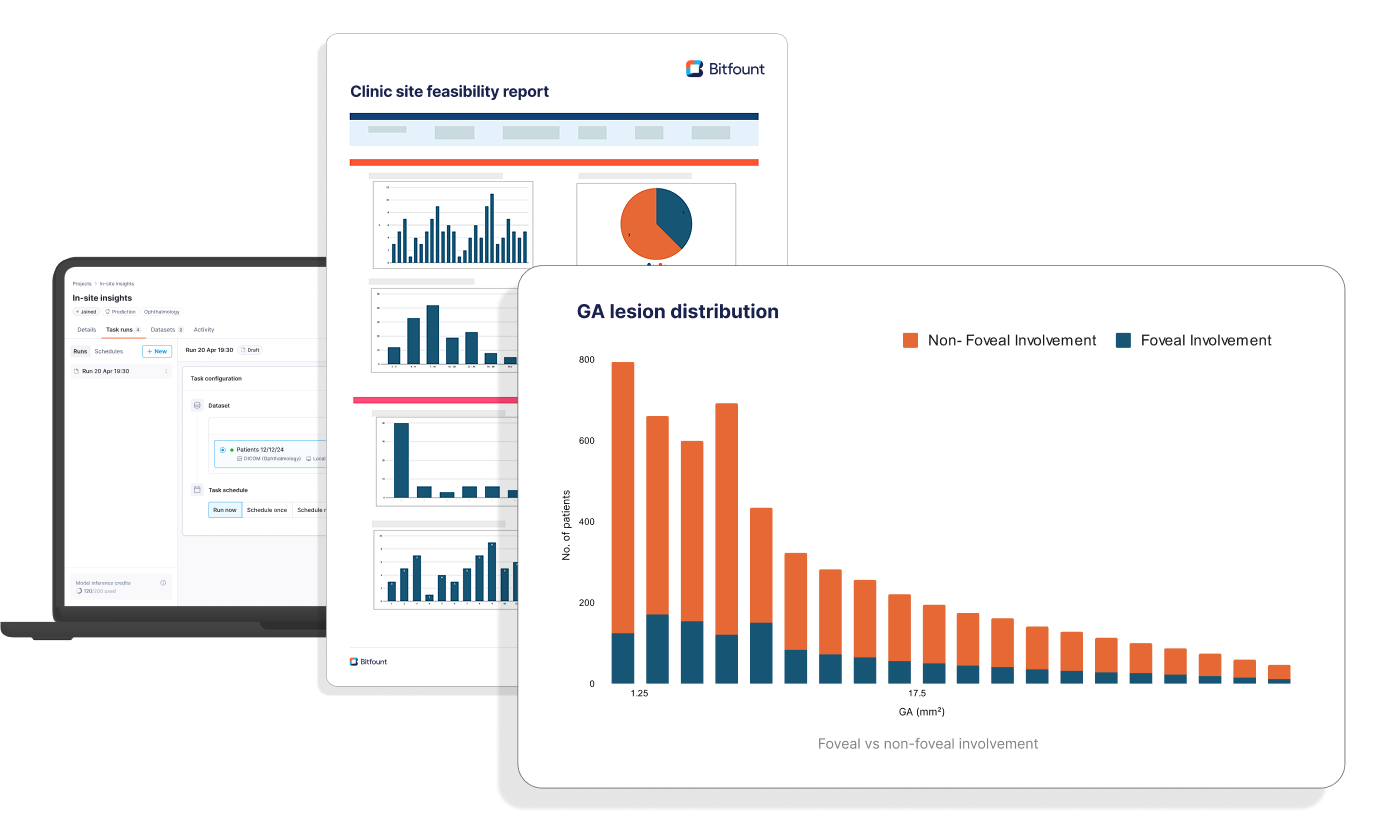
In-Site Insights™ automates the quantification of imaging data into interpretable metrics such as:
- Disease prevalence segmented by GA, CNV, dry/wet AMD, and DME, etc
- GA lesion size, count and growth dynamics, including foveal and non-foveal involvement
- Population-level demographics, including age distribution and image volume by patient and eye
- Imaging acquisition trends over time, broken down by day and month
For investigators, this translates into a clear understanding of the disease landscape within their own clinic.
For CROs, it means faster, evidence-based feasibility assessments.
For sponsors, it offers quantifiable confidence in site selection and recruitment forecasting.
Additionally, for CROs and sponsors, it serves as a standardised, unbiased site-level analysis, ensuring that insights are derived using the same validated algorithms and allowing direct comparison of data across all participating sites.
The Bitfount platform performs the AI analysis using local computation, which means that all analysis occurs within the clinic’s IT environment, meaning no data is ever shared with Bitfount or the model provider.
Discover how In-Site Insights™ can transform your clinic’s research readiness. Explore the platform, request a demo, or speak to Bitfount’s team about deploying secure, on-premise analytics for your clinics and studies.
Contact us: https://www.bitfount.com/contact
In-Site Insights™ is a population-level analytics tool intended for research and feasibility assessment purposes only. It is not a medical device and is not intended for clinical diagnosis or direct patient care.
























%20(12).png)
.png)
.png)
.png)
.png)
